NCERT Exemplar Class 11 Chemistry Chapter 6 Thermodynamics are part of NCERT Exemplar Class 11 Chemistry. Here we have given NCERT Exemplar Class 11 Chemistry Chapter 6 Thermodynamics.
NCERT Exemplar Class 11 Chemistry Chapter 6 Thermodynamics
Multiple Choice Questions
Single Correct Answer Type
Q1. Thermodynamics is not concerned about.
(a) energy changes are involved in a chemical reaction.
(b) the extent to which a chemical reaction proceeds.
(C) the rate at which a reaction proceeds.
(d) the feasibility of chemical reaction.
Sol: (c) Thermodynamics is not concerned with the rate at which a reaction proceeds. The rate of reaction is dealt with by kinetics.
Also See: NCERT Solutions for Class 11 Chemistry Chapter 5 States of Matter
Q2. Which of the following statements is correct?
(a) The presence of reacting species in a covered beaker is an example of an open system.
(b) There is an exchange of energy as well as a matter between the system and the surroundings in a closed system.
(c) The presence of reactants in a closed vessel made up of copper is an example of a closed system.
(d) The presence of reactants in a thermos flask or any other closed insulated vessel is an example of a closed system.
Sol: (c) For a closed vessel made up of copper, no matter can be exchanged between the system and the surroundings but energy exchange can occur through its walls.
Q3. The state of gas can be described by quoting the relationship between
(a) pressure, volume, temperature
(b) temperature, amount, pressure
(c) amount, volume, temperature
(d) pressure, volume, temperature, amount
Sol: (d) State of a system can be described by state functions or state variables which are pressure, volume, temperature and amount of the gas (PV= nRT).
Q4. The volume of gas is reduced to half from its original volume. The specific
heat will.
(a) reduce to half (b) be doubled
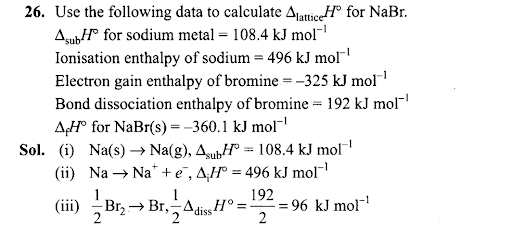
(c) remain constant (d) increase four times
Sol: (c) The specific heat of a substance is the heat required to raise the temperature of 1 gram of a substance by one degree (1 K or 1 °C). It is an intensive property and is independent of the volume of the substance.
Q5. During complete combustion of one mole of butane, 2658 kJ of heat is released. The thermochemical reaction for the above change is
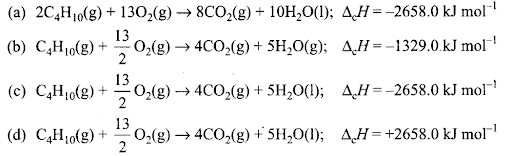
Sol: (c) Exothermic reaction for the combustion of one mole of butane is represented as:
![]()
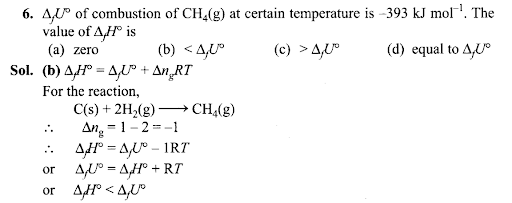
Q7. In an adiabatic process, no transfer-of heat takes place between the system and surroundings. Choose the correct option for free expansion of an ideal gas under the adiabatic condition from the following.
![]()
Sol: (c) For free expansion w = 0 For adiabatic process q = 0 From first law of thermodynamics,
∆U=q + w
= 0 + 0 = 0
Since there is no change of internal energy, hence temperature will also remain constant, i.e., ∆T = 0
Q8. The pressure-volume work for an ideal gas can be calculated by using the expression
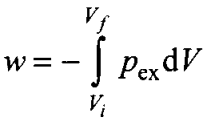
The work can also be calculated from the pV
plot by using the area under the curve within the specified limits. When an ideal gas is compressed (a) reversibly or (b) irreversibly from Vi to Vf, choose the correct option.
(a) w (reversible) = w (irreversible)
(b) w (reversible) < w (irreversible)
(c) w (reversible) > w (irreversible)
(d) w (reversible) = w (irreversible) + pex. ∆V
Sol: (b) w (reversible) < w (irreversible)
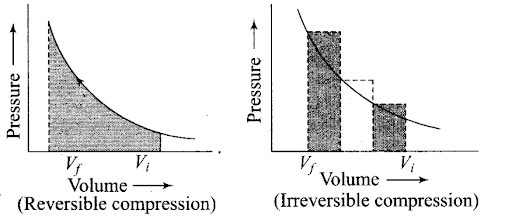
The area under the curve is greater in irreversible compression than that of reversible compression.
Q9. The entropy change can be calculated by using the expression ∆S = q rev / T. When water freezes in a glass beaker, choose the correct statement amongst the following:
When water freezes in a glass beaker, choose the correct statement amongst the following:
(a) ∆S(system) decreases but ∆S(surroundings) remains the same.
(b) ∆S(system) increases but ∆S(surroundings) decreases.
(C) ∆S(system) decreases but ∆S(surroundings) increases.
(d) ∆S(system) decreases but ∆S(surroundings) also decreases.

Q10. On the basis of thermochemical equations (i), (ii), and (iii), find out which of the algebraic relationships given in options (a) to (d) is correct.

Q11. Consider the reactions given below. On the basis of these reactions find out which of the algebraic relations given in options (a) to (d) is correct?
(i) C(g) + 4H(g) → CH4(g); ∆rH= kJ mol-1
(ii) C(graphite, s) + 2H2(g) → CH4(g); ∆rH = y kJ mol 1
(a) x = y (b) x = 2y (c)x >y (d)x< y
Sol: (c) x > y because same bonds are formed in reactions (i) and (ii) but bonds
between reactant molecules are broken only in reaction (ii). As energy is absorbed when bonds are broken, energy released in reaction (i) is greater than that in reaction (ii).
Q12. The enthalpies of elements in their standard states are taken as zero. The enthalpy of formation of a compound
(a) is always negative
(b) is always positive
(c) maybe positive or negative
(d) is never negative
Sol:(c) Heat of formation of a compound may be positive or negative, e.g.,

Q13. Enthalpy of sublimation of a substance is equal to
(a) enthalpy of fusion + enthalpy of vapourization
(b) enthalpy of fusion
(c) enthalpy of vapourization
(d) twice the enthalpy of vapourization.
Sol: (a) Enthalpy of sublimation of a substance is equal to enthalpy of fusion + enthalpy of vapourisation.
Sublimation is direct conversion of solid to vapour, i.e., solid → vapour
Writing in two steps, we have solid → liquid → vapour.
Solid → liquid requires enthalpy of fusion.
Liquid →vapour requires enthalpy of vapourisation
Also See: NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
Q14. Which of the following is not correct?
(a) ∆G is zero for a reversible reaction.
(b) ∆G is positive for a spontaneous reaction
(c) ∆G is negative tor a spontaneous reaction
(d) ∆G is positive for a non-spontaneous reaction.
Sol:(b) ∆G gives a criterion for spontaneity at constant pressure and temperature.
(i) If ∆G is negative (< 0). the process is spontaneous.
(ii) If ∆G is positiv e (> 0). the process is non-spontaneous.
(iii) If ∆G is zero then reaction is at equilibrium.
More than One Correct Answer Type
Q15. Thermodynamics mainly deals with
(a) interrelation of various forms of energy and their transformation front one from to another.
(b) energy changes in the processes which depend only on initial and final states of the microscopic system containing a few molecules.
(c) how and at what rate these energy transformations are carried out.
(d) the system in equilibrium state or moving from one equilibrium state to another equilibrium state.
Sol: (a, d) Thermodynamics deals with interrelation of various forms of energy and their transformation into each other. It also deals with thermal or mechanical equilibrium. However, if does not tell anything about the rate of reaction.
Q16. In an exothermic reaction, heat is evolved, and system loses heat to the surroundings. For such system
(a) qP will be negative
(b) ∆γHwill be negative
(c) qp will be positive
(d) ∆γHwill be positive.
Sol:(a, b) For an exothermic reaction, qp = -ve, ∆γH = -ve
Q17. The spontaneity means, having the potential to proceed without the assistance of external agency. The processes which occur spontaneously are
(a) flow of heat from colder to warmer body.
(b) gas in a container contracting into one comer.
(c) gas expanding to fill the available volume.
(d) burning carbon in oxygen to give carbon dioxide.
Sol:(c, d) Gas expands or diffuses in available space spontaneously, e.g., leakage of cooking gas gives smell of ethyl mercaptan spontaneously. Moreover, burning of carbon to C02 is also spontaneous.
Q18. For an ideal gas. the work of reversible expansion under isothermal condition 1.0 mol of an ideal gas is expanded isothermally and reversibly to ten times of its original volume, in two separate experiments. The expansion is carried out at 300 K and at 600 K respectively. Choose the correct option.
can be calculated by using expression w = -nRT In Vf / Vi A sample containing
(a) Work done at 600 K is 20 times the work done at 300 K.
(b) Work done at 300 K is twice the work done at 600 K
(c) Work done at 600 K is twice the work done at 300 K.
(d) ∆U= 0 in both cases.
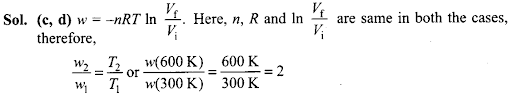
i.e., work done at 600 K is twice the work done at 300 K. Since each case involves isothermal expansion of an ideal gas, there is no change in internal energy, i.e., ∆U = 0.
Q19. Consider the following reaction between zinc and oxygen and choose the correct options out of the options given below:
2Zn(s) + 02(g) → 2ZnO(s); ∆H=-693.8 kJ mol-1
(i) The enthalpy of two moles ZnO is less than the total enthalpy of two moles of Zn and one mole of oxygen by 693.8 kJ.
(ii) The enthalpy of two moles of ZnO is more than the total enthalpy of two moles of Zn and one mole of oxygen by 693.8 kJ.
(iii) 8 kJ mol -1 energy is evolved in the reaction.
(iv) 693.8 kJ mol-1 energy is absorbed in the reaction.
Short Answer Type Questions
Q20. 18.0 g of water completely vapourises at 100°C and 1 bar pressure and the enthalpy change in the process is
40.79 kJ mol-1. What will be the enthalpy change for vapourising two moles of water under the same conditions? What is the standard enthalpy of vapourization for water?
Sol: Enthalpy of a reaction is the energy change per mole for the process.
18 g of H20 = 1 mole (∆Hvap = 40.79 kJ moE1)
Enthalpy change for vapourising 2 moles of H20 = 2 x 40.79 = 81.58 kJ ∆H°vap = 40.79 kJ mol -1

Q21. One mole of acetone requires less heat to vapourise than 1 mol of water. Which of the two liquids has higher enthalpy of vapourisation?
Sol: Water has higher enthalpy of vapourisation. (∆Hr)water > (∆Hr)acetone
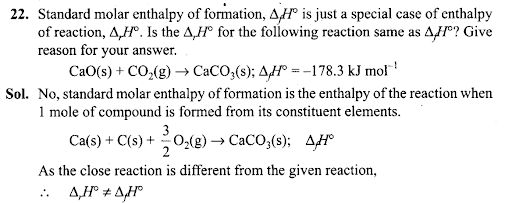
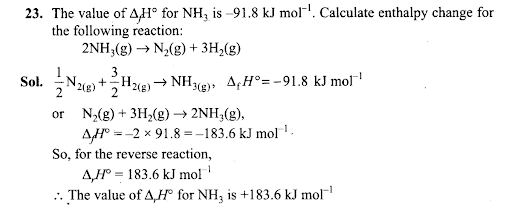
Q24. Enthalpy is an extensive property. In general, if enthalpy of an overall reaction A→B along one route is ∆rH and ∆rH1, ∆rH2, ∆rH3 …. represent enthalpies of intermediate reactions leading to product B. What will be the relation between ∆rH for overall reaction and ∆rH1, ∆rH2….. etc. for intermediate reactions.
Sol: By Hess’s law ∆rH = ∆rH1+ ∆rH2 + ∆rH3……………………
Q25. The enthalpy of atomisation for the reaction CH4(g) → C(g) + 4H(g) is 1665 kJ mol-1. What is the bond energy of C – H bond?
Sol: CH4 → C + 4H, ∆H= + 1665 kJ mol-1 ,
Bond energy of (C – H) bond = 1665/4 =416.2 kJ mol

Q27. Given that ΔH= 0 for mixing of two gases. Explain whether the diffusion of these gases into each other in a closed container is a spontaneous process or not?
Sol: It is a spontaneous process. Although enthalpy change is zero but randomness or disorder (ΔS) increases and ΔS is positive. Therefore, in equation, ΔG = ΔH – TΔS, the term TΔS will be negative. Hence ΔG will be negative.
Q28. Heat has randomising influence on a system and temperature is the measure of average chaotic motion of particles in the system. Write the mathematical relation which relates these three parameters.
Sol: Heat has randomising influence on a system and temperature is the measure of average chaotic motion of particles in the system. The mathematicalrelation which relates these three parameters is ΔS = q rev/ T
Here, ΔS = change in entropy ^
qrcv = heat of reversible reaction ‘
T = temperature
Q29. Increase in enthalpy of the surroundings is equal to decrease in enthalpy of the system. Will the temperature of system and surroundings be the same when they are in thermal equilibrium?
Sol: Yes, when the system and the surroundings are in thermal equilibrium, their temperatures are same.
Q30. At 298 K, Kp for the reaction N204(g)⇌ 2N02(g) is 0.98. Predict whether the reaction is spontaneous or not.
Sol: ΔrG° = -RT ln Kp
= -RT ln (0.98)
Since In (0.98) is negative
.’. ΔrG° is positive
=> the reaction is non spontaneous
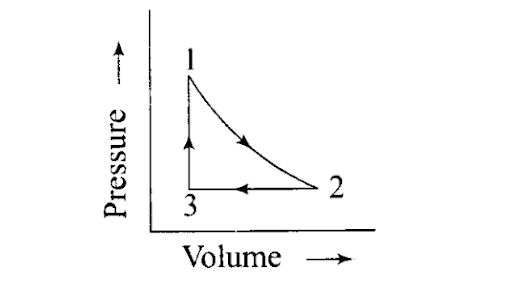
Q31. A sample of 1.0 mol of a monoatomic ideal gas is taken through a cyclic process of expansion and compression as shown in the figure. What will be the value of ΔHfor the cycle as a whole?
Sol: For a cyclic process, ΔH = 0
Q32. The standard molar entropy of H2O(l) is 70 J K-1 mol-1. Will the standard molar entropy H20(s) be more, or less than 70 J K -1 mol-1?
Sol: The standard molar entropy of H20 (1) is 70 J K-1 mol-1. The solid form of H20 is ice. In ice, molecules of H20 are less random than in liquid water. Thus, molar entropy of H20 (s) < molar entropy of H20 (1). The standard molar entropy of H20 (s) is less than 70 J K 1 mol-1.
Q33. Identify the state functions and path functions out of the following: enthalpy, entropy, heat, temperature, work, free energy.
Sol: State functions: Enthalpy, entropy, temperature, free energy Path functions: Heat, work
Q34. The molar enthalpy of vapourisation of acetone is less than that of water. Why?
Sol: Molar enthalpy of vapourisation is more for water due to hydrogen bonding between water molecules.
Q35. Which quantity out of ΔrG and ΔrG° will be zero at equilibrium?
Sol: Gibbs energy for a reaction in which all reactants and products are in standard state. ΔrG° is related to the equilibrium constant of the reaction as follows
ΔrG = ArG° + RT In K
At equilibrium, 0 = ΔrG° + RT In A– ({ΔrG = 0) or ΔrG° =-RT lnK
ΔrG° = 0 when K= 1
For all other values of K, ArG° will be non-zero.
Q36. Predict the change in internal energy for an isolated system at constant volume.
Sol: For an isolated system w = 0, q = 0
Since ΔU= q + w = 0 + 0 = 0, ΔU= 0
Q37. Although heat is a path function but heat absorbed by the system under certain specific conditions is independent of path. What are those conditions? Explain.
Sol: At constant volume
q = ΔU + (-w)
-w = pΔ q = AU + pΔV
ΔV = 0 (at constant volume)
Hence, qv = ΔU + 0 = ΔU= change in internal energy At constant pressure, qp = AU + pΔV
Since ΔU + pΔV=ΔH
=> qp = ΔH change in enthalpy
Hence, at constant volume and at constant pressure, heat change is a state function because it is equal to ΔU and ΔH respectively which are state functions.
Q38. Expansion of a gas in vacuum is called free expansion. Calculate the work done and the change in internal energy when 1 litre of ideal gas expands isothermally into vacuum until its total volume is 5 litre.
Sol: During free expansion, external pressure is zero, so Work done, w = -pextΔV
= -0(5 – 1) = 0
Since the gas is expanding isothermally, therefore, q = 0
ΔU = q + w =0+0=0
Q39. Heat capacity (CP) is an extensive property but specific heat (c) is an intensive property. What will be the relation between Cp and c for 1 mol of water?
Sol: For water, molar heat capacity = 18 x Specific heat or
Cp = 18 x c
But, specific heat,
C = 4.18 J g-1 K-1 Heat capacity,
Cp = 18 x 4.18 JK 1 = 75.24 JK-1
Q40. The difference between Cp and Cv can be derived using the empirical relation H = U + pV. Calculate the difference between Cp and Cv for 10 moles of an ideal gas.
Sol: Given that, Cv = heat capacity at constant volume,
Cp = heat capacity at constant pressure Difference between Cp and Cv is equal to gas constant (R).
.’. Cp – Cv = nR (where, n = no. of moles)
= 10 x 8.314 = 83.14J
Q41. If the combustion of 1 g of graphite produces 20.7 kJ of heat, what will be molar enthalpy change? Give the significance of sign also.
Sol: Molar enthalpy change for graphite (ΔH)
= enthalpy change for 1 g x molar mass of C = -20.7×12 = -2.48 x 102 kJ mol-1
Since the sign of ΔH = -ve, it is an exothermic reaction.
Q42. The net enthalpy change of a reaction is the amount of energy required to break all the bonds in reactant molecules minus amount of energy required to form all the bonds in the product molecules. What will be the enthalpy change for the following reaction?
H2(g) + Br2(g)→2HBr(g)
Given that bond energy of H2, Br2 and HBr is 4.35 kJ mol-1,192 kJ mol-1 and 368 kJ mol -1 respectively.